“The real innovation here is the ability to change multiple aspects of somebody’s life after they have experienced a spinal cord injury.”
“The real innovation here is the ability to change multiple aspects of somebody’s life after they have experienced a spinal cord injury.”
REPRINTED FROM CRAIN’S CLEVELAND BUSINESS
A pioneer in the field of rehabilitation, the MetroHealth Rehabilitation Institute helps patients regain as much physical and cognitive function as possible so that they can get back to the things that matter to them. And, as one of only 18 federally designated Spinal Cord Injury Model System Centers in the nation, the MetroHealth Rehabilitation Institute is leading the way to restore function, social participation, and quality of life for people with significant spinal cord injuries.
Founded in 1953, the MetroHealth Rehabilitation Institute has yet again been ranked by U.S. News & World Report as one of the best in the nation for the care of patients recovering from complex conditions such as stroke, traumatic brain injury, and traumatic spinal cord injury.
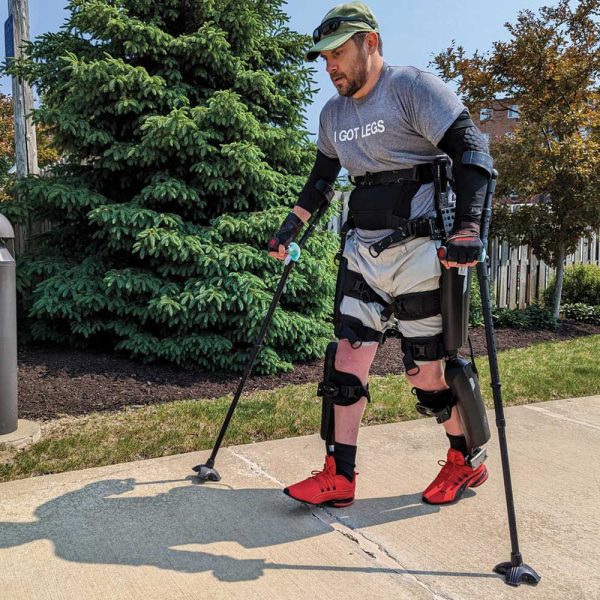
One of the rehabilitation research topics MetroHealth focuses on is called functional electrical stimulation, or FES. FES applies small electrical pulses to paralyzed muscles to restore or improve function. Electrical impulses stimulate the muscle to produce its usual movement, helping to assist with breathing, grasping, transferring, standing, and walking. FES devices can be implanted to permanently replace lost functions, which means movement is restored in ways that were previously impossible. MetroHealth continues to use FES research to find new ways to use the technology.
From Research To Real Life
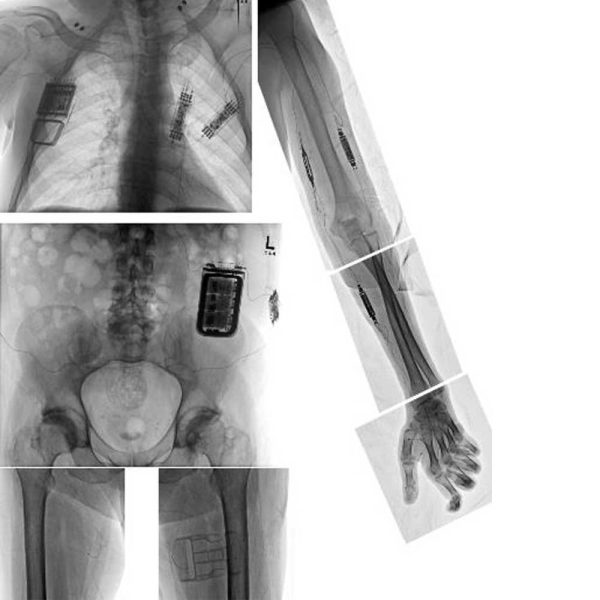
At the forefront of FES technology is the Networked Neuroprosthesis (NNP) device, developed by Kevin Kilgore, PhD, and Hunter Peckham, PhD. First implanted at MetroHealth in 1986, Kilgore and Peckham evolved the NNP to coordinate stimulation of multiple muscle groups to produce complex movement and even restore organ function.
Through Kilgore and Peckham’s work, the device has gone through several iterations, with the newest version being implanted beginning in 2016. The pair is continuing to build and improve upon their life-changing design to restore function for people who experience paralysis. “Someone who has had a spinal cord injury at the level of their neck is essentially paralyzed from the neck or shoulders down,” says Dr. Kilgore. “Systems in the past would provide only one function — like hand grasp — at a time, while the current NNP device provides multiple functions at the same time.”
Developments like these move research on how to restore function in specific areas to real life, where multiple functions are necessary to thrive.
“We always wanted to develop a system that could provide as much function as possible,” says Dr. Kilgore. “That’s what led to us trying to design this… creating something that could give somebody hand capability, enable them to stand, and allow them to cough, and so forth all simultaneously—that’s what drove us.”
Richard Wilson, MD, a physiatrist and the Interim Chair of Physical Medicine and Rehabilitation, says MetroHealth takes pride in the researchers who are working on improving the lives of those with disabilities.
“The real innovation here is the ability to change multiple aspects of somebody’s life after they have experienced a spinal cord injury,” says Dr. Wilson. “We try to get as much of their body working back to as close to normal as possible and restore multiple aspects of the functions that have been lost.”
Research That Goes The Extra Mile
Functional Electrical Stimulation—the technology that drives the NNP—has promising applications beyond the device itself, and research on FES applications is already showing results at MetroHealth.
Adam Gorlitsky is a para-athlete who is used to moving fast. While Gorlitsky holds the Guinness World Record for the fastest marathon completed in a robotic walking device, he competed in the 2023 Cleveland Marathon to highlight the groundbreaking research that allowed him to empty his bladder more quickly. After developing a severe kidney infection as the result of his spasming bladder, MetroHealth enrolled him in a clinical trial to implant an FES device.
“It completely changed my life,” says Gorlitsky, explaining that prior to receiving the implant, he had to empty his bladder manually using a catheter in a time-consuming process that made him prone to urinary tract infections and incontinence. With his FES device, he is able to empty his bladder in three to five minutes.
That device is part of an ongoing study that seeks to determine whether the participant’s existing implanted device is more effective when different frequencies are applied using an external controller. The aim is to determine if the FES device could help to empty the bladder more quickly and without a catheter if the frequency of the electrical stimulation were increased, showing the technology’s promise beyond the Networked Neuroprothesis device.
While being able to walk in his robotic walking device is exciting, Dr. Kilgore knows through his work with people with paralysis that improved bladder function is a higher priority among patients.
“One of the things MetroHealth prides itself on is focusing on what matters most to people with spinal cord injuries,” says Dr. Kilgore.
Research Dollars Increase Access
The potential within the development of the NNP has brought external research dollars, including a $12.5 million grant from the National Institutes of Health, as well as support from the Department of Defense; the National Institute on Disability, Independent Living, and Rehabilitation Research; the State of Ohio, and private philanthropic funds.
And, true to MetroHealth’s focus on health equity, a portion of the funds is devoted to creating resources to make the NNP open source so that any health researcher can build upon it.
“We’re giving away the knowledge to any researcher who wants it,” Dr. Wilson says. “The goal is to expand what this sort of technology can do in order to get more minds working on it.”
Research funds also allow MetroHealth to gather a larger team of junior investigators and engineers to collaborate on the continued advancement of the technology. Consistent with MetroHealth’s standing as an academic medical center, training this next generation of researchers will support the growing momentum of projects and help ensure that MetroHealth continues to move forward as a leader in rehabilitation technology.
“Our goal is to get FDA approval and make the NNP more accessible,” says Dr. Kilgore. “Right now anyone can get it, but they have to come to Cleveland. Ultimately, we want to have this system available throughout the world so that people who have spinal cord injury can benefit from it. We’re trying to do whatever we can to help people become more independent.”
For more information about the MetroHealth Rehabilitation Institute’s research:
Visit our website MetroHealth Rehabilitation Institute.
If you are interested in learning more about the clinical study for people with spinal cord injuries, visit ClinicalTrials.gov and search for study NCT05863754,
or email UE.FES.ClinicalTrials@gmail.com. For more information about the open source NNP implant, visit cosmiic.org.
Contributors: